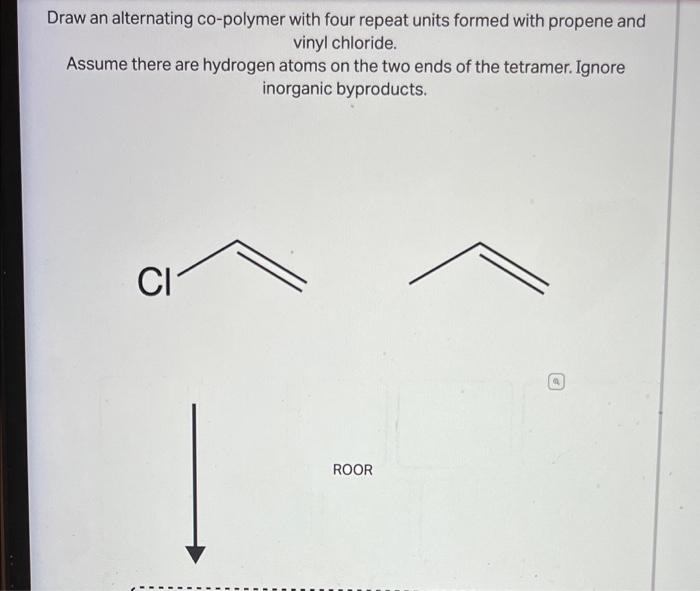
ISBN: 9781305080485 Author: John E. McMurry Publisher: John E. McMurry Chapter31: Synthetic Polymers Section31.SE: Something Extra Problem 21AP See similar textbooks Related questions Question Transcribed Image Text: Draw a tetramer of this alternating copolymer. HO но.
A) Calculated absorption bands of two planarized DA-tetramers,… | Download Scientific Diagram
Question Solved step-by-step Draw a tetramer of this alternating copolymer. OH OH OH HO heat Select to Draw Draw a tetramer of this alternating copolymer. OH OH OH HO heat Select to Draw Submitted by Carolyn A. Feb. 10, 2023 02:07 p.m. Video Answer Solved by verified expert Video Player is loading. Play Video Current Time 0:00 / Duration 0:00
Source Image: chegg.com
Download Image
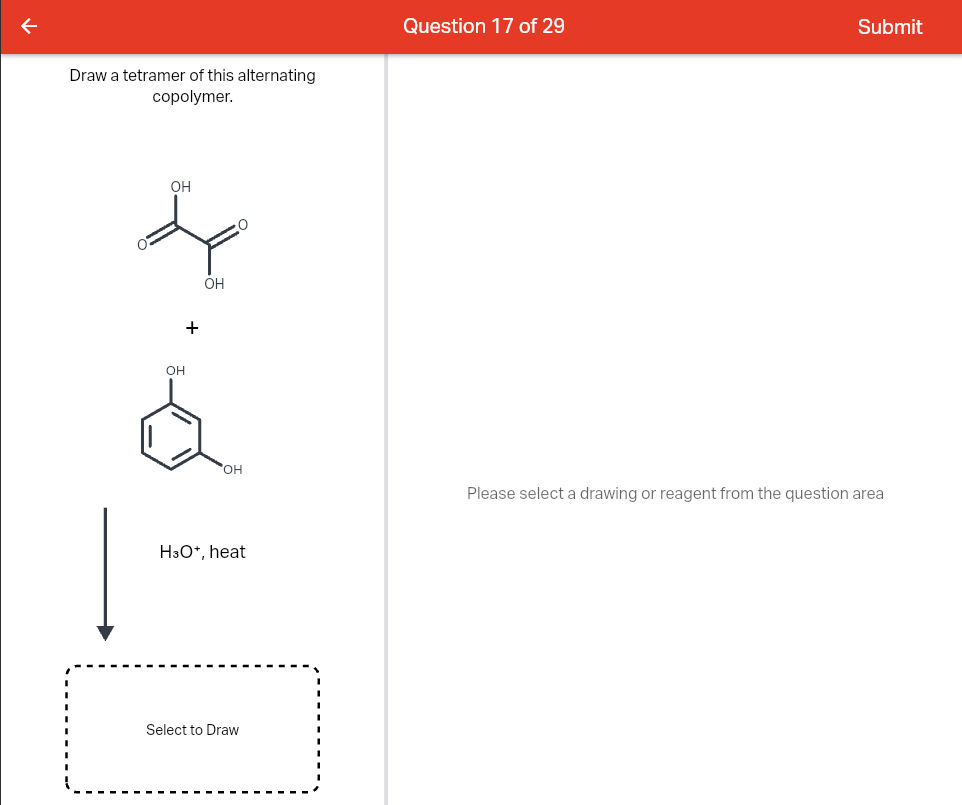
Question Transcribed Image Text: Draw a tetramer of this alternating copolymer. НО HO. + H3O+, heat OH OH Д А Expert Solution Trending now This is a popular solution! Step by step Solved in 3 steps with 1 images SEE SOLUTION Check out a sample Q&A here Knowledge Booster Learn more about Types of Polymers on the Basis of Physical Properties

Source Image: pinterest.com
Download Image
p-Block Elements – Definition, Properties, Uses and Examples – WBBSE Solutions Nylon 6 is not an alternating co-polymer like nylon 66. It’s just a polymer. And it isn’t made from a difunctional monomer like nylon 66 or proteins. Instead, it’s made from a cyclic amide, sometimes called a lactam. To polymerize, the lactam has to break open into a linear form, and the lactam monomers end up enchained head-to-tail.
Source Image: chegg.com
Download Image
Draw A Tetramer Of This Alternating Copolymer
Nylon 6 is not an alternating co-polymer like nylon 66. It’s just a polymer. And it isn’t made from a difunctional monomer like nylon 66 or proteins. Instead, it’s made from a cyclic amide, sometimes called a lactam. To polymerize, the lactam has to break open into a linear form, and the lactam monomers end up enchained head-to-tail. Jul 25, 2022Exercise 10.3.1 10.3. 1. Homopolymers are made with a single monomer and are made up of identical repeating units. Copolymers is made when two or more different monomers are polymerized together to create a polymer with variable repeating units. For example the monomers hexafluoropropene and vinylidene fluoride can be polymerized together to
Solved Draw a tetramer of this alternating copolymer. Please | Chegg.com
Grammar checker Expert proofreading This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. Polyesters are versatile polymers widely used in everyday materials, and one common type is polyethy… Draw a tetramer of this alternating copolymer. Q Synthesis of methacrylic acid-acrylonitrile alternating copolymer using an extremely bulky methacrylate and the sequence-dependent thermal reaction behaviors – ScienceDirect

Source Image: sciencedirect.com
Download Image
One-Pot Preparation of Methacrylate/Styrene Alternating Copolymers via Radical Copolymerization and Alcoholysis Modification: Sequence Impacts on Glass Transition Temperature | ACS Polymers Au Grammar checker Expert proofreading This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. Polyesters are versatile polymers widely used in everyday materials, and one common type is polyethy… Draw a tetramer of this alternating copolymer. Q

Source Image: pubs.acs.org
Download Image
A) Calculated absorption bands of two planarized DA-tetramers,… | Download Scientific Diagram ISBN: 9781305080485 Author: John E. McMurry Publisher: John E. McMurry Chapter31: Synthetic Polymers Section31.SE: Something Extra Problem 21AP See similar textbooks Related questions Question Transcribed Image Text: Draw a tetramer of this alternating copolymer. HO но.
Source Image: researchgate.net
Download Image
p-Block Elements – Definition, Properties, Uses and Examples – WBBSE Solutions Question Transcribed Image Text: Draw a tetramer of this alternating copolymer. НО HO. + H3O+, heat OH OH Д А Expert Solution Trending now This is a popular solution! Step by step Solved in 3 steps with 1 images SEE SOLUTION Check out a sample Q&A here Knowledge Booster Learn more about Types of Polymers on the Basis of Physical Properties
Source Image: wbbsesolutions.guide
Download Image
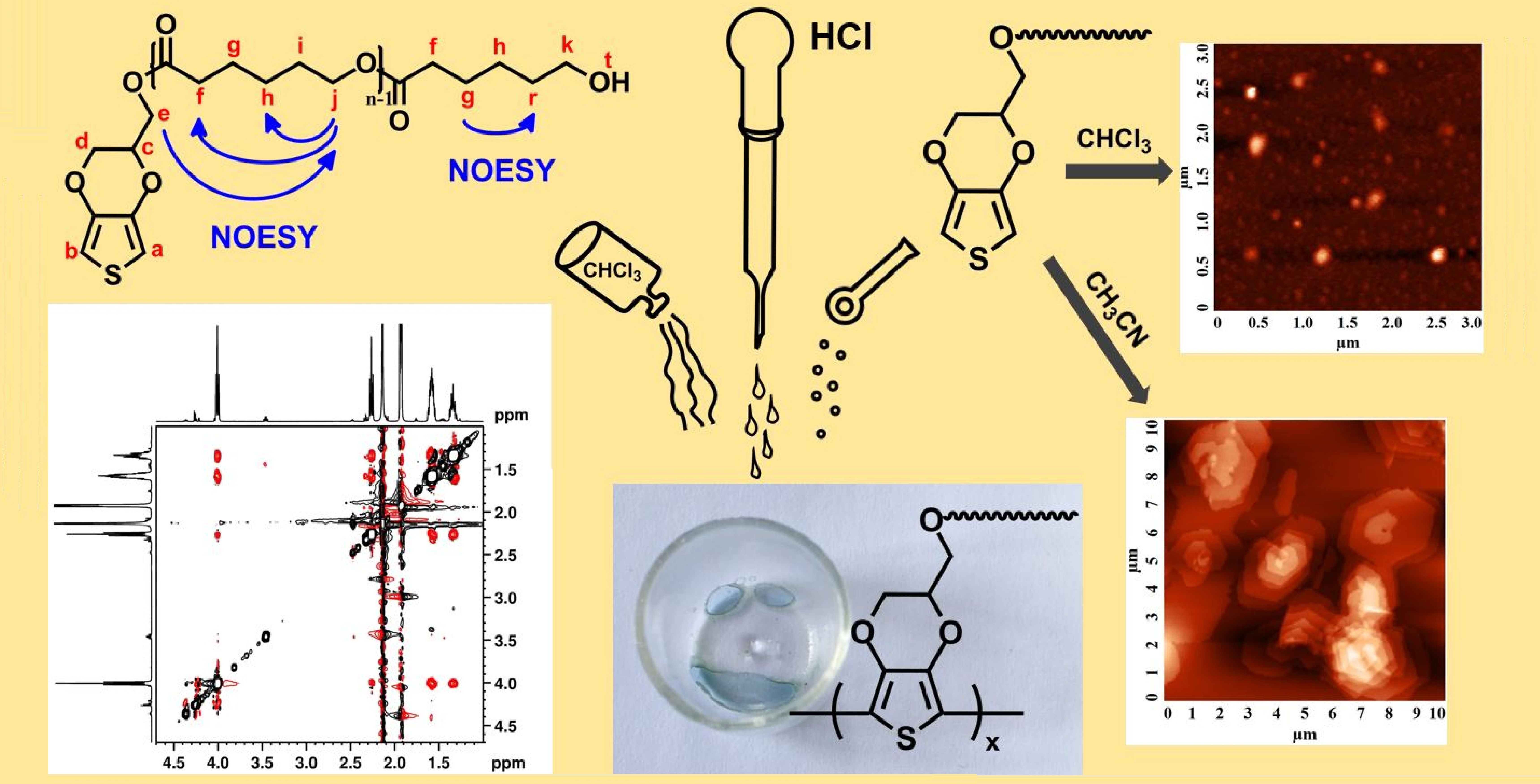
Polymers | Free Full-Text | 3,4-Ethylenedioxythiophene (EDOT) End-Group Functionalized Poly-ε-caprolactone (PCL): Self-Assembly in Organic Solvents and Its Coincidentally Observed Peculiar Behavior in Thin Film and Protonated Media Question Transcribed Image Text: H3O+, heat Drawing Version: 1.115.1 + production Transcribed Image Text: Draw a tetramer of this alternating copolymer. H₂N OH + OH 0 Q Ⓒ NH₂ Expert Solution Step by step Solved in 4 steps with 2 images SEE SOLUTION Check out a sample Q&A here Knowledge Booster Learn more about
Source Image: mdpi.com
Download Image
PPT – ADDITION POLYMERS PowerPoint Presentation, free download – ID:910400 Nylon 6 is not an alternating co-polymer like nylon 66. It’s just a polymer. And it isn’t made from a difunctional monomer like nylon 66 or proteins. Instead, it’s made from a cyclic amide, sometimes called a lactam. To polymerize, the lactam has to break open into a linear form, and the lactam monomers end up enchained head-to-tail.
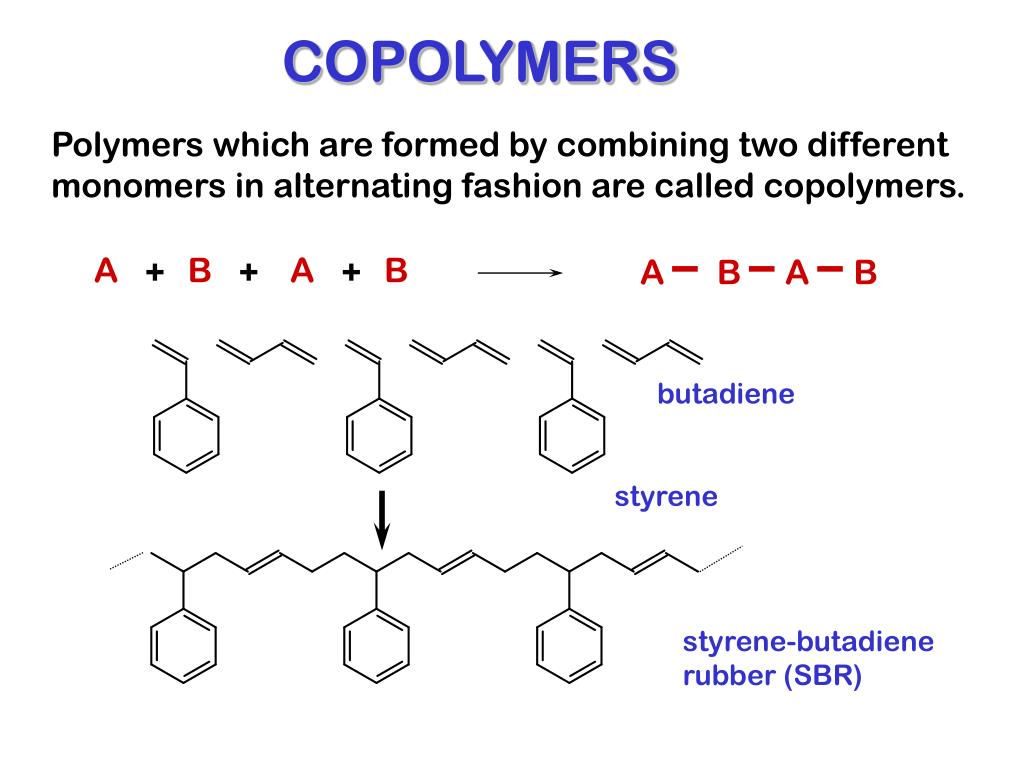
Source Image: slideserve.com
Download Image
Molecules considered in this paper: an oligomer of a block polymer… | Download Scientific Diagram Jul 25, 2022Exercise 10.3.1 10.3. 1. Homopolymers are made with a single monomer and are made up of identical repeating units. Copolymers is made when two or more different monomers are polymerized together to create a polymer with variable repeating units. For example the monomers hexafluoropropene and vinylidene fluoride can be polymerized together to

Source Image: researchgate.net
Download Image
One-Pot Preparation of Methacrylate/Styrene Alternating Copolymers via Radical Copolymerization and Alcoholysis Modification: Sequence Impacts on Glass Transition Temperature | ACS Polymers Au
Molecules considered in this paper: an oligomer of a block polymer… | Download Scientific Diagram Question Solved step-by-step Draw a tetramer of this alternating copolymer. OH OH OH HO heat Select to Draw Draw a tetramer of this alternating copolymer. OH OH OH HO heat Select to Draw Submitted by Carolyn A. Feb. 10, 2023 02:07 p.m. Video Answer Solved by verified expert Video Player is loading. Play Video Current Time 0:00 / Duration 0:00
p-Block Elements – Definition, Properties, Uses and Examples – WBBSE Solutions PPT – ADDITION POLYMERS PowerPoint Presentation, free download – ID:910400 Question Transcribed Image Text: H3O+, heat Drawing Version: 1.115.1 + production Transcribed Image Text: Draw a tetramer of this alternating copolymer. H₂N OH + OH 0 Q Ⓒ NH₂ Expert Solution Step by step Solved in 4 steps with 2 images SEE SOLUTION Check out a sample Q&A here Knowledge Booster Learn more about